The release of the excess phase from the supersaturated solution at the low temperatures leads to the increase in the strength and hardness of the alloy. This is called dispersion hardening or aging. During aging, there is usually a lot of precipitation, evenly distributed throughout the alloy. Especially, a lot of them during exposure at room temperature. In this case, the decomposition of the supersaturated solution occurs slowly and is termed natural aging.
With the heating of the alloy, the precipitation intensifies and is called artificial aging. At the initial stages of natural aging in the supersaturated solution (Fig. 1, a), there are regions enriched with the dissolved component, called the Guinier-Preston zones (Fig. 1, b). They have the form of disks with a diameter of tens and a thickness of several interatomic distances.
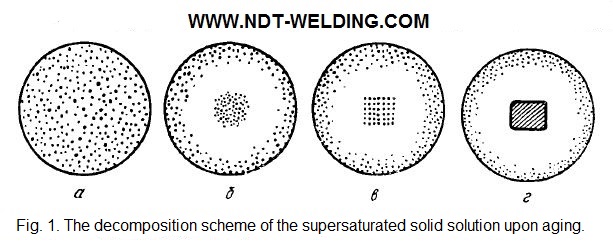
Due to diffusion, they can coalesce and unite or dissipate. At first glance, the formation of the Guinier-Preston zones is energetically unjustified and should lead to an increase in the thermodynamic potential of the alloy.
According to Fig. 2a, the thermodynamic potential of the homogeneous supersaturated solution (Za) is lower than that of the inhomogeneous solution (Zб), consisting of the zones of the composition Св enriched with component B and a solution of composition Сг.
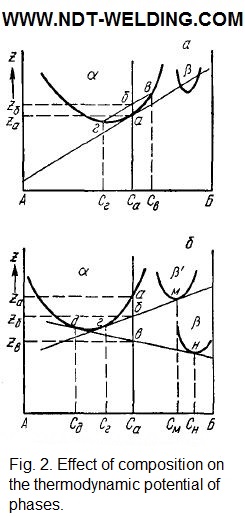
This increase in the thermodynamic potential (ΔZ = Zб — Za) can be compensated by other processes that reduce the thermodynamic potential by more than ΔZ. Such reduction can be caused by eliminating the defectiveness of the initial solution, for example, reducing the concentration of excess vacancies. Excessive vacancies interact with atoms of the dissolved component. Their binding energy is approximately 1 eV. Therefore, in a supersaturated quenched solution, vacancy-dissolved-vacancy complexes or a double vacancy-the dissolved atom-are stable. The transfer of excess vacancies to the drains is accompanied by the diffusion of component B in the same direction. In the place of vacancy, the dissolved atoms segregate and form the Guinier-Preston zones. Using this representation, they explain the high rate of formation of zones. They do not have a specific structure and are distorted sections of the solid solution enriched with the dissolved component.






