In the solid metal the atoms are aligned in even lines, rows and layers, forming three-dimensional crystalline structures. By definition, the metals have the crystalline structure. It is obvious that any reasoning about «crystallization» as the cause of the destruction of metal has no basis for any reason. When solidified, the metal always acquires the crystalline structure. The formation of a fracture surface, which is mistakenly termed «crystal-like» because of its appearance, is typical for cases of fatigue or the brittle fracture.
The smallest number of atoms that form the structure that gives a complete picture of their ordered arrangement is called the elementary cell. One should pay attention to the fact that elementary cells do not exist as independent units, but have common atoms with neighboring elementary cells in the three-dimensional matrix.
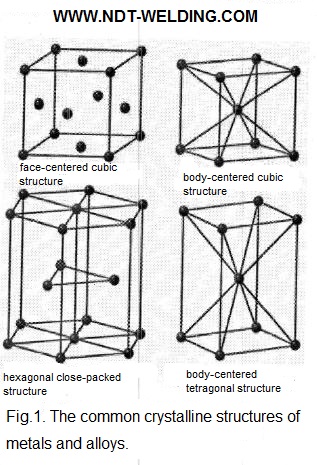
The most common crystal structures, or phases, include a body-centered cubic structure, a face-centered cubic structure, a body-centered tetragonal structure, and a hexagonal close-packed structure. These structures are shown in Fig.1.
Some metals, such as iron, are in the same solid phase at the room temperature, and in another solid phase – at elevated temperature. The transition of the solid metal from one phase to another with the temperature change is called an allotropic or solid-phase transition. The metal crystal having different structures, but the same chemical composition, is called allotropic.
The volume-centered cubic unit cell can be described as the cube in each of the eight corners of which is located on the atom. Another atom is located in the center of the cell. The common metals with the body-centered structure include iron, various grades of carbon steel, chromium, molybdenum and tungsten.
The face-centered cubic unit cell can be represented as the cube having one atom at each of the eight angles, as well as one atom at the center of each of the six faces. The common metals having a face-centered cubic structure include aluminum, copper, nickel, and various grades of austenitic stainless steel.
The body-centered tetragonal unit cell resembles a body-centered cubic elementary cell with the atom in the center, one of whose axes lengthens, giving the structure a rectangular shape. The martensite is the phase of steel, which is formed as a result of quenching by abrupt cooling and has the body-centered tetragonal structure.
The hexagonal close-packed elementary cell is a hexagonal prism. This form can be described as two hexagons forming the upper and lower parts of the prism. In the center and in each of the corners of the hexagon is located one atom. Three atoms located in the corners of the triangle are located between the upper and lower hexagons. The common metals with the hexagonal close-packed structure include zinc, cadmium and magnesium.






