The diffusionless transformation can occur as a result of the disordered atomic transitions (it is called «massive»). It is possible to combine the ordered, cooperative rearrangement of the lattice with the diffusion redistribution of components between the initial and the resulting solid solutions. Which of the indicated mechanisms of polymorphic transformations is realized depends on the magnitude of the change in the thermodynamic potential, on the ratio of cooling rates, the diffusion and lattice rearrangement, and on the structure of the solid solutions in the considered system (see Fig. 1).
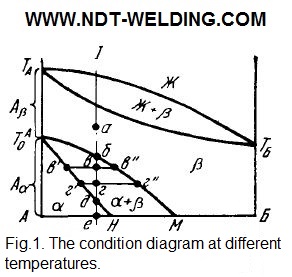
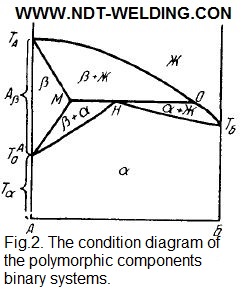
The B component B and the β-modification of component A are isomorphic. If the B component and the a-modification of the A component are isomorphic, then the condition diagram has the different form (see Fig. 2).
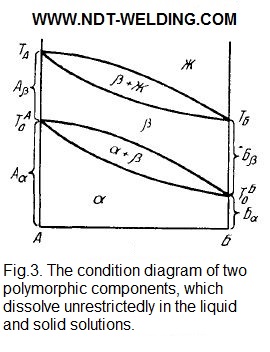
In Fig. 3 shows the condition diagram of the two polymorphic components that dissolve unrestrictedly in the liquid and solid solutions.
With the metals limited solubility in the solid condition, the equilibrium diagrams are complicated. In Fig. 4, a, b show the phase diagrams of the systems of components whose high-temperature modifications are isomorphic and dissolve into each other completely, forming the β solution, while low-temperature ones dissolve in the limited way, forming the α1 and α2 solutions.
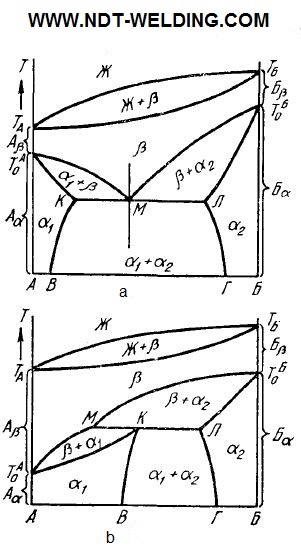
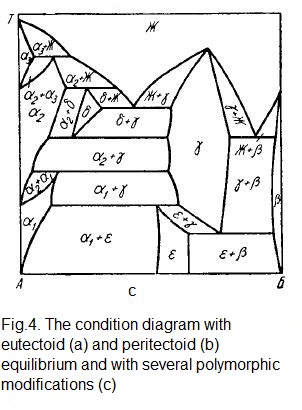
In the diagrams below the solidus line, there are three regions of single-phase (α1, α2 and β) and three two-phase (α1 + α2; α1 + β; β + α2) conditions. In both systems, three-phase equilibria are possible. The first of these, α1 + α2 + β, similar to the eutectic one, is called the eutectoid (Fig. 4, a, the KMЛ line), the second is the peritectoid equilibrium β + α1 + α2 (Fig. 4, b, the МКЛ line).






