The impurities are important in the polymorphous transformations. The nature of the influence of impurities is related to their solubility in the metal. If the impurity does not dissolve in the solid metal, the polymorphic transformation in the alloys is similar, as we described earlier. In this case, as for pure metal, it is possible to indicate a certain equilibrium temperature of both modifications, which does not depend on the composition of the alloy.
In Fig. 1, the condition diagram of the double system which component A has a polymorphic point (То) above the eutectic temperature is provided, and the component B is lower than the eutectic (То).
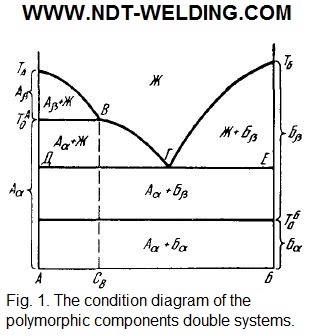
The Aβ↔Aα transformations are possible only in alloys whose composition lies in the concentration range of АСв. Upon cooling to temperatures below ТАо, the previously evolved crystals of the β-modification of component A undergo a polymorphic transformation into Аα. The polymorphic transformation in these alloys can also be avoided if accelerated cooling achieves supercooling of the liquid to temperatures below the point of ТАо, when it becomes possible to directly crystallize Аα. The polymorphic transformations of Бα↔Бβ are possible in all alloys of this system and occur after solidification.
If the introduction of the insoluble impurity has almost no effect on the molecular pattern of repackaging the atoms, the position changes with the formation of solid solutions. Let us first consider the polymorphous transformations in a system of components that are unboundedly soluble in the liquid and solid conditions (Fig. 2).
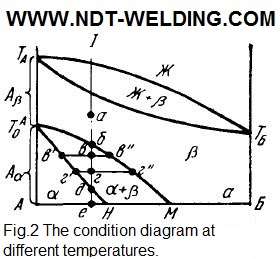
The area below the solidus line is divided into three sections: АТАоН,, where the alloys are stable in the single-phase state α, which is a solid solution of the α-modification of component A and the non-polymorph component of Б; ТАоТАТББМ, where the alloys are in the β state, representing the solid solution of the β-modification of component A and component B; ТАоМН where the two-phase state α + β is stable. At temperatures above ТАо (but below the solidus line), all alloys consist of β-phase crystals, whose thermodynamic potential is less than α (Fig. 3).
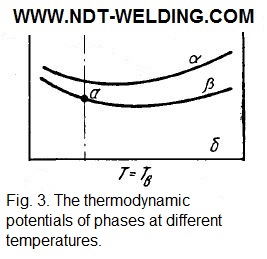
With decreasing temperature, the curves of the changes in the thermodynamic potential of the α- and β-solutions approach each other in certain places. At temperatures below ТАо, the curves intersect (Fig. 4).
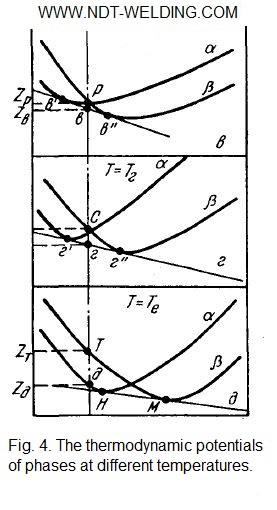
At these temperatures, the condition of the alloys varies with the composition. For example, at Т = Тв (Fig. 4 b), the alloys of the interval АСв‘ are in a single-phase state α, which provides them with a minimum of the thermodynamic potential. The alloys of the Св”Б interval are stable in the single-phase state of the β-solid solution. The alloys of the interval are stable in the two-phase α + β state, their thermodynamic potential lies on the segment of the tangent в’в”.
Thus, in the alloys of the МБ interval, at all temperatures, from room temperature to solidus, the stable phase is the β solution. The all polymorphic transformation β → α is tested by the alloys of the AH interval. Alloys, whose composition lies between the points H and M, are partially transformed.






