The transformation hardening of crystalline phases is a promising method of hardening metals. At temperatures above T0, the low-temperature modification of α is transformed into a high-temperature modification of β. In metals that have undergone an ordered polymorphic transformation upon cooling, heating can cause an inverse α → β transition without violating the coherence of the lattices. The characteristic feature of direct and reverse shear polymorphic transformation is a large temperature hysteresis. Along with the ordered polymers, the normal polymorphic transformation is also possible: the polygonization and recrystallization processes can strongly affect the structure of the heated metal.
The recrystallization is complicated if the polymorphic metal has three or more modifications. In Fig. 1 (bottom) shows the change in the thermodynamic potential of three modifications of a single metal.
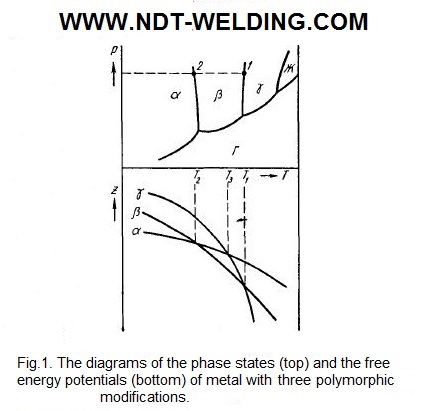
At T1 and T2, respectively, γ + β- and β + α-modifications are in equilibrium. At T> T1, under equilibrium conditions, the metal consists of γ-modification crystals. At temperatures below T1, the polymorphic transformations will occur. Depending on the degree of hypothermia, they occur in different ways.
The γ modification is super cooled to temperatures in the interval T1-T3, can be transformed into β-modification. The cooling to temperatures below T2 can cause the conversion of β → α.
If the original γ-modification is super cooled to temperatures below T3, it becomes possible to separate both the β and α modification, since their formation leads to the decrease in the thermodynamic potential of the metal. In the temperature range T3-T2, the γ → β conversion would lead to the greater decrease in the thermodynamic potential than the conversion of γ → α, and it would seem that the γ → β transition is preferable. However, it may be that the formation of metastable α-modification is kinetically more favorable at the temperatures considered, which may be due, for example, to the great similarity of the lattices α and β or to a smaller difference in their specific volumes. As a result, the probability of nucleation of the α-phase and the rate of its growth will be greater than for the β-phase. Under these supercoolings, the simultaneous separation of both α and β phases is possible. However, with a long exposure in the temperature range T3-T2, the metastable α-phase will pass to the stable β-phase. If, after the formation of α-phase, the metal rapidly cools down to temperatures below T2, the α-phase is stable and β is metastable. Under these conditions, the single-phase stable equilibrium is achieved by a complete transition of β- to α-phase.
In some cases, at the high cooling rate, the γ-modification is super cooled to the temperatures below T2. At such temperatures, the polymorphic transformation can lead to the formation of α and β crystals. The quantitative relationship between the phases formed is determined by the kinetic conditions. In this case, the β-phase, being metastable, can then be converted into a stable α-phase.






